At A Glance
INDUSTRY
Manufacturing
SOLUTION
Docuflo DMS and BPMS
Overview
A leading manufacturing company specializing in industrial and consumer rubber products sought to improve efficiency in its Quality Assurance (QA) processes, particularly in handling document approvals, customer complaints, and internal audits. Previously, the client relied on paper-based
and email-driven requests, leading to delays, inefficiencies, and compliance risks. Multi-layer approvals and manual tracking complicated workflows, making it difficult to maintain document consistency, traceability, and regulatory compliance. To address these challenges, the client implemented Business Process Management System (BPMS) for workflow automation and Docuflo Document Management System (DMS) for secure document storage and compliance tracking, improving efficiency, transparency, and process control across multiple QA functions.
The Objective
The client faced challenges in QA workflows, due to manual approvals, causing lost requests and inefficiencies. Multi-layer sign-offs led to delays, while version control issues created compliance risks. Limited visibility required frequent follow-ups, delaying document distribution and tracking.
The Solution
The client faced challenges in QA workflows, due to manual approvals, causing lost requests and inefficiencies. Multi-layer sign-offs led to delays, while version control issues created compliance risks. Limited visibility required frequent follow-ups, delaying document distribution and tracking.
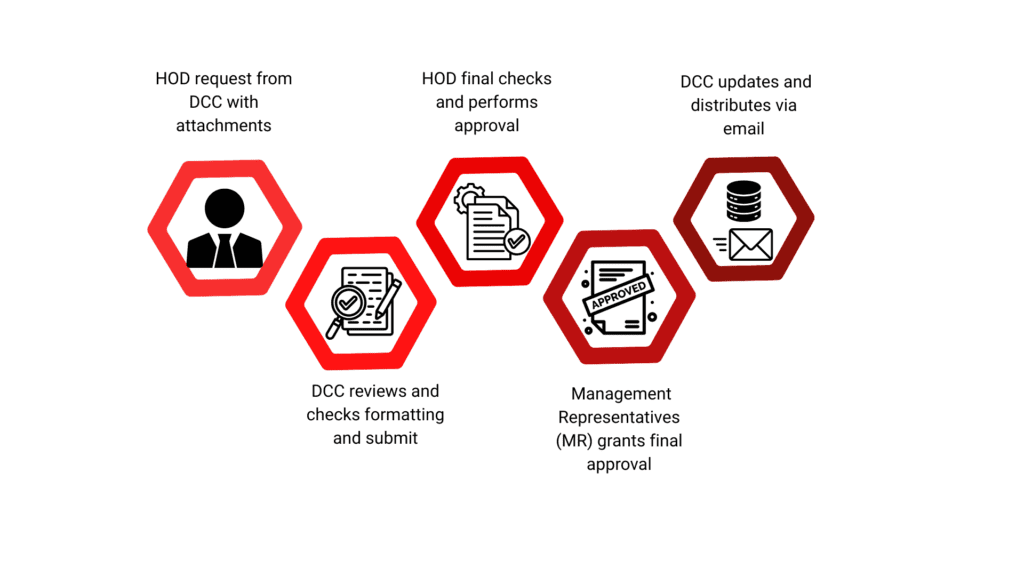
The above is a sample workflow for a Document Change Request by plants
The company adopted BPMS to automate approval workflows within the QA department, specifically for Document Change Requests, Customer Complaints, and Internal Audits, reducing manual intervention and approval delays. Meanwhile, Docuflo DMS ensures secure, version controlled document management, serving as a centralized repository for QA documents, SOPs, and compliance records. Role-based access control restricts document handling to authorized personnel, while audit-ready compliance tracking logs and timestamps maintains full traceability of all document actions, ensuring regulatory compliance. This integration enhances efficiency, security, and visibility across the QA department’s processes.
By implementing BPMS and Docuflo DMS, the company has modernized its QA processes for better efficiency, compliance, and visibility, thus setting a strong foundation for future workflow
automation and operational improvements.
The Benefits
The solution implemented by InfoConnect for the QA department significantly enhanced operational efficiency and regulatory readiness, delivering the following benefits:
1. Faster Approvals
Automation removes manual routing delays and streamlines the approval process. With digital workflows in place, approvers are notified instantly, and documents move through multi-level approvals much faster. This accelerated processing shortens the time required for critical actions such as change requests and audit responses, enabling quicker execution and reduced operational lag.
2. Better Compliance
Audit trails are automatically generated and maintained for every document action, including edits, approvals, and version updates. This complete traceability supports audit readiness and helps meet strict regulatory requirements. The system ensures that document history is easily accessible, verifiable, and tamper-proof, significantly reducing compliance risks and administrative overhead.
3. Increased Efficiency
Workflow automation reduces the need for manual follow-ups and repetitive administrative tasks. Employees are automatically notified of pending actions, minimizing delays and boosting productivity. By freeing up staff from routine paperwork, the organization enables teams to focus on strategic and high-priority tasks, driving overall departmental efficiency.
4. Stronger Security
Role-based access controls ensure that only authorized personnel can view, modify, or approve specific documents. This safeguards sensitive QA and compliance records from unauthorized access or tampering. Combined with version control and audit logs, the system enhances document integrity and ensures the confidentiality of business-critical information.
